
Comparing and Contrasting Delirium and Acute Encephalopathy
Last week I wrote about querying for the type of acute encephalopathy, and I’d like to continue this week. Please know, when I use the
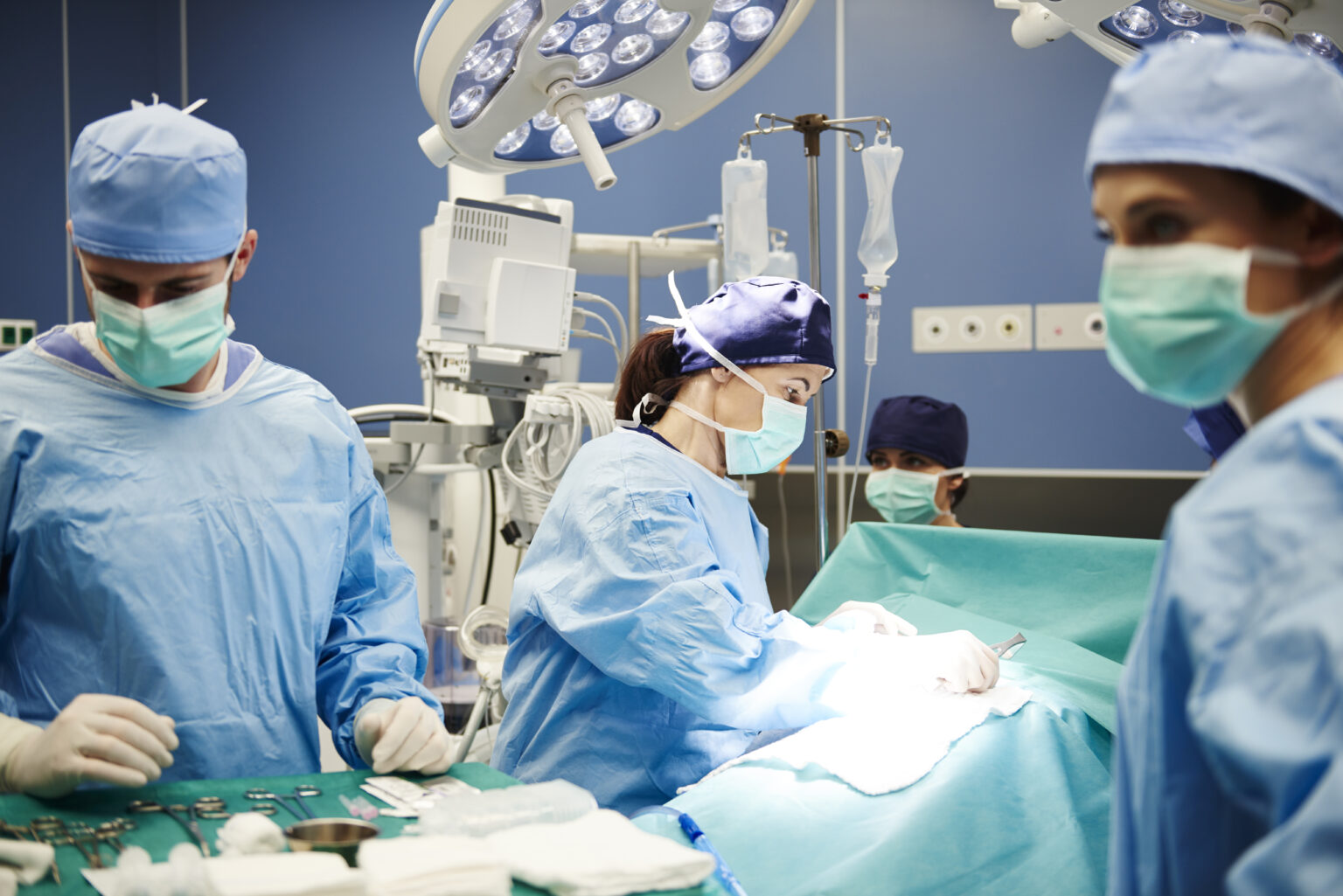
Chimeric Antigen Receptor T-cell (CAR T-cell) immunotherapy is a topic near to my heart, as a loved one is about to undergo this treatment plan. This article focuses on this immunotherapy approach.
CAR T-cell therapy can be used for certain types of blood cancers that have relapsed or are refractory to traditional treatment methods, for example B-cell acute lymphoblastic leukemia (ALL), diffuse large B-Cell lymphoma, follicular lymphoma, high-grade B-cell lymphoma, mantle cell lymphoma, multiple myeloma, and primary mediastinal large B-cell lymphoma. Traditional methods of treatment include chemotherapy or radiation therapy, but CAR T-cell therapy falls into the immunotherapy class, and is showing promise. This is because science uses our body’s T-cells to create a cancer-killing army to go in and attack the cancer cells at their core.
But how do our T-cells do that? Here’s the process: T-Cells are extracted from a person’s white blood cells via a process called leukapheresis. The cells are then genetically modified by adding a special receptor called chimeric antigen receptors (CAR) and then reinfused back into the patient. The new T-cells with the added receptor will now begin to multiply via normal cell duplication, and then detect and attack the cancer cells. Since T-cells are responsible for finding abnormal cells in the body, once identified, they organize an army to attack the intruder, and call in reinforcements by activating other parts of the immune system to help in the fight. This is why treatment with CAR T-cell therapy is considered immunotherapy: because it uses the patient’s own immune system to fight cancer cells.
For coding purposes, when a patient is admitted for this type of therapy, we would use Z51.12 (Encounter for antineoplastic immunotherapy) as the principal diagnosis (PDx), followed by the cancer code. CAR T-cell therapy is not used as first-line therapy, and it does come with potential for severe adverse effects. Genetic modifications to the T-cells can be accomplished using the patients’ own T-cells or donor T-cells, also known as autologous or allogeneic. However, the risk for graft versus host disease or adverse reactions is greater when using allogeneic or “off-the-shelf” CAR T-cells than with autologous cells.
ICD-10-CM has developed a specific immune effector cellular therapy complication code if adverse reactions occur. This code can be found at T80.82 (complication of immune effector cellular therapy), with X being a placeholder for the sixth character and the appropriate seventh character added to indicate initial, subsequent, or sequela for the type of encounter. The two most common side effects, as noted by The Cleveland Clinic, are cytokine release syndrome (CRS) and neurological issues.
CRS is an inflammatory process that can lead to fevers, low blood pressure, shortness of breath, or heart and lung issues, and can be graded on a scale from 1 to 5, depending on the severity. If a patient was to develop CRS, that would be captured using D89.83 (cytokine release syndrome), with the appropriate sixth character to indicate the grade, if known. This code would follow T80.82 to specify the type of complication from an immune effector cellular therapy code. Examples of a few neurological side effects could include aphasia, balance problems, confusion, and seizures, which can be a sign of developing toxicity. Immune effector cell-associated neurotoxicity syndrome (ICANS) can present itself with those neurological symptoms, with the most serious being cerebral edema and/or intracerebral hemorrhage. ICANS can also be graded by severity on a scale of 1 to 5, and would be coded G92.0 (immune effector cell-associated neurotoxicity syndrome), with the appropriate fourth character to indicate the grade, if known. This code would also follow T80.82. Coders should look very carefully for any adverse effects as this will impact the APR-DRG Severity of Illness (SOI) and/or Risk of Mortality (ROM).
There isn’t just one ICD-10-PCS code to capture CAR T-cell therapy, as there are several different types that cater to the specific blood cancer. For instance, idecabtagene vicleucel is a B-cell maturation antigen-directed genetically modified autologous CAR T-cell therapy for the treatment of adult patients with multiple myeloma. It’s coded to XW043K7 (introduction of Idecabtagene Vicleucel Immunotherapy into central vein, percutaneous approach, new technology group 7) when administered via central vein. There are seven specific PCS codes for CAR T-cell therapy, which determine the sixth character of device, but all will drive the DRG or APR-DRG into the CAR T-cell and other immunotherapies group.
In summary, CAR T-cell therapy represents an important advancement in cancer treatment, offering hope to patients with relapsed or refractory blood cancers when traditional therapies fall short. While the process is complex and carries risks of serious adverse effects, accurate coding and documentation are essential to capture both the therapy and its potential complications. By understanding the science, the coding guidelines, and the clinical implications, healthcare professionals and coders can work together to ensure that patients receive the best possible care while maintaining precise reporting.


Last week I wrote about querying for the type of acute encephalopathy, and I’d like to continue this week. Please know, when I use the

Behavioral healthcare, encompassing mental health and substance use disorder services, has moved from the margins of health policy to the forefront of national priorities. Payers,
Please log in to your account to comment on this article.

Sepsis remains one of the most frequently denied and contested diagnoses, creating costly revenue loss and compliance risks. In this webcast, Angela Comfort, DBA, MBA, RHIA, CDIP, CCS, CCS-P, provides practical, real-world strategies to align documentation with coding guidelines, reconcile Sepsis-2 and Sepsis-3 definitions, and apply compliant queries. You’ll learn how to identify and address documentation gaps, strengthen provider engagement, and defend diagnoses against payer scrutiny—equipping you to protect reimbursement, improve SOI/ROM capture, and reduce audit vulnerability in this high-risk area.

Only ICD10monitor delivers what you need: updates on must-know changes associated with the FY26 IPPS, including new ICD-10-CM/PCS codes, CCs/MCCs, and MS-DRGs, plus insights, analysis and answers to your questions from two of the country’s most respected subject matter experts.

This third session in our 2026 IPPS Masterclass will feature a review of FY26 changes to the MS-DRG methodology and new technology add-on payments (NTAPs), presented by nationally recognized ICD-10 coding expert Christine Geiger, MA, RHIA, CCS, CRC, with bonus insights and analysis from Dr. James Kennedy.

This second session in our 2026 IPPS Masterclass will feature a review the FY26 changes to ICD-10-PCS codes. This information will be presented by nationally recognized ICD-10 coding expert Christine Geiger, MA, RHIA, CCS, CRC, with bonus insights and analysis from Dr. James Kennedy.

During this essential RACmonitor webcast Michael Calahan, PA, MBA Certified Compliance Officer, will clarify the rules, dispel common misconceptions, and equip you with practical strategies to code, document, and bill high-risk split/shared, incident-to & critical care E/M services with confidence. Don’t let audit risks or revenue losses catch your organization off guard — learn exactly what federal auditors are looking for and how to ensure your documentation and reporting stand up to scrutiny.

Learn how to navigate the proposed elimination of the Inpatient-Only list. Gain strategies to assess admission status, avoid denials, protect compliance, and address impacts across Medicare and non-Medicare payors. Essential insights for hospitals.

RACmonitor is proud to welcome back Dr. Ronald Hirsch, one of his most requested webcasts. In this highly anticipated session, Dr. Hirsch will break down the complex Two Midnight Rule Medicare regulations, translating them into clear, actionable guidance. He’ll walk you through the basics of the rule, offer expert interpretation, and apply the rule to real-world clinical scenarios—so you leave with greater clarity, confidence, and the tools to ensure compliance.

Bring your questions and join the conversation during this open forum series, live every Wednesday at 10 a.m. EST from June 11–July 30. Hosted by Chuck Buck, these fast-paced 30-minute sessions connect you directly with top healthcare experts tackling today’s most urgent compliance and policy issues.
Prepare for the 2025 CMS IPPS Final Rule with ICD10monitor’s IPPSPalooza! Click HERE to learn more
Get 15% OFF on all educational webcasts at ICD10monitor with code JULYFOURTH24 until July 4, 2024—start learning today!
CYBER WEEK IS HERE! Don’t miss your chance to get 20% off now until Dec. 2 with code CYBER24